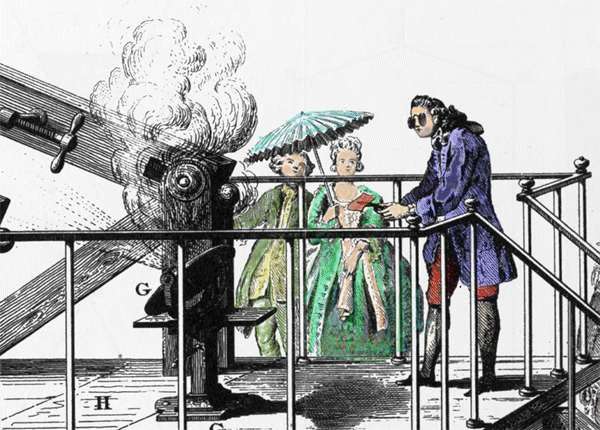
Antoine Lavoisier conducting an experiment related combustion generated by amplified sun light.
On Nov 1, 1772, French chemist Antoine Lavoisier [1] reported in a note to the Secretary of the French Academy of Sciences about the role of “air” in the combustion process. It required five more years of experiments, before in 1777, Lavoisier was ready to propose a new theory of combustion that excluded phlogiston,[4] which according to the prevailing theories of the time was part of every matter and responsible for the combustion process.
“Here, then: a revolution [in science and chemistry] has taken place in an important part of human knowledge since your departure from Europe… I will consider this revolution to be well advanced and even completely accomplished if you range yourself with us. …After having brought you up to date on what is happening in chemistry, it would be well to speak to you about our political revolution. We regard it as done and without any possibility of return to the old order.”
– Antoine Lavoisier, Letter to Benjamin Franklin (Feb 2, 1790)
Modern Chemistry started with Lavoisier
Modern chemistry started with Antoine Lavoisier, as we have already learned in a previous article about Lavoisier’s life and achievements [1]. Today, we focus on one particular subject, Lavoisier`s theory of combustion. During late 1772 Lavoisier turned his attention to the phenomenon of combustion, the topic on which he was to make his most significant contribution to science. In the times of Lavoisier, processes such as combustion and rusting, have not been related to oxidation like nowadays. In 1667, German chemist Johann Joachim Becher [4] had published his so-called phlogiston theory. The theory attempted to explain all burning processes. in general, substances that burned in air were said to be rich in a substance called phlogiston. The fact that combustion soon ceased in an enclosed space was taken as clear-cut evidence that air had the capacity to absorb only a finite amount of phlogiston. When air had become completely phlogisticated it would no longer serve to support combustion of any material, nor would a metal heated in it yield a calx; nor could phlogisticated air support life. Breathing was thought to take phlogiston out of the body. Thus, Becher described phlogiston as a process that explained combustion through a process that was opposite to that of oxygen.
Lavoisier’s Experiments
Antoine Lavoisier reported the results of his first experiments on combustion in a note to the Academy on 20 October 1772, in which he reported that when phosphorus burned, it combined with a large quantity of air to produce acid spirit of phosphorus, and that the phosphorus increased in weight on burning. In a second sealed note deposited with the Academy a few weeks later on November 1, 1772, Lavoisier extended his observations and conclusions to the burning of sulfur and went on to add that “what is observed in the combustion of sulfur and phosphorus may well take place in the case of all substances that gain in weight by combustion and calcination: and I am persuaded that the increase in weight of metallic calces is due to the same cause.” Although Lavoisier now realized that combustion actually involved air, the exact composition of air at that time was not clearly understood.
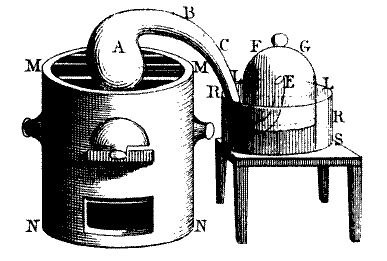
Antoine Lavoisier’s phlogiston experiment. Engraving by Mme Lavoisier in the 1780s taken from Traité Élémentaire de Chimie
Exchange with Priestley
In the spring of 1774 Lavoisier carried out experiments on the calcination of tin and lead in sealed vessels which conclusively confirmed that the increase in weight of metals in combustion was due to combination with air. But the question remained about whether it was combination with common atmospheric air or with only a part of atmospheric air. In October the eminent English natural philosopher Joseph Priestley visited Paris [2], where he met Lavoisier and told him of the air which he had produced by heating the red calx of mercury with a burning glass and which had supported combustion with extreme vigor. Priestley at this time was unsure of the nature of this gas, but he felt that it was an especially pure form of common air.
Lavoisier’s Theory of Combustion
In Paris, the intrigued Lavoisier repeated Priestley’s experiment with mercury and other metal calces. He eventually concluded that common air was not a simple substance. Instead, he argued, there were two components: one that combined with the metal and supported respiration and the other an asphyxiant that did not support either combustion or respiration. By 1777, Lavoisier was ready to propose a new theory of combustion that excluded phlogiston. Phlogiston remained the dominant theory until the 1780s when Lavoisier showed that combustion requires a gas that has mass (oxygen) and could be measured by means of weighing closed vessels. Lavoisier’s chemical research between 1772 and 1778 was largely concerned with developing his own new theory of combustion. In 1783 he read to the academy his famous paper entitled Réflexions sur le phlogistique (Reflections on Phlogiston), a full-scale attack on the current phlogiston theory of combustion.
To promote the breakthrough of his oxidation theory, Lavoisier organized a stage play in June 1789, which he performed at the Arsenal. On the stage appeared the flammable phlogiston, it was accused of serious crimes by Oxygène. The advocate, Professor Stahl, defended the phlogiston. Finally, phlogiston was sentenced to death by fire. Madame Lavoisier was the sacrificial priestess in this.
Michael Hoepfner, Lecture 8 – Combustion Reactions (Jan. 26, 2018) [5]
Related work and further reading:
- [1] Modern Chemistry started with Lavoisier, SciHi Blog
- [2] Joseph Priestley and the Discovery of Oxygen, SciHi Blog.
- [3] The Chemical Revolution of Antoine-Laurent Lavoisier, International Historic Chemical Landmark, Dedicated June 8, 1999, at the Académie des Sciences de l’Institut de France in Paris, France.
- [4] Johann Joachim Becher and the Phlogiston Theory of Combustion, SciHi Blog
- [5] Michael Hoepfner, Lecture 8 – Combustion Reactions (Jan. 26, 2018), Michael Hoepfner @ youtube
- [6] Works by or about Antoine Lavoisier at Internet Archive
- [7] Oeuvres de Lavoisier (Works of Lavoisier) at Gallica BnF in six volumes.
- [8] Antoine Lavoisier at Wikidata
- [9] Antoine Lavoisier Timeline via Wikidata






Pingback: Whewell’s Gazette: Year 2, Vol. #17 | Whewell's Ghost