Robert Wilhelm Bunsen (1811-1899)
On March 30, 1811, German chemist Robert Wilhelm Bunsen was born. Bunsen investigated emission spectra of heated elements, and discovered caesium (in 1860) and rubidium (in 1861) with the physicist Gustav Kirchhoff.[6] He developed several gas-analytical methods, was a pioneer in photochemistry, and did early work in the field of organoarsenic chemistry. With his laboratory assistant, Peter Desaga, he developed the Bunsen burner, an improvement on the laboratory burners then in use. The Bunsen–Kirchhoff Award for spectroscopy is named after Bunsen and Kirchhoff.
“A chemist who’s not a physicist is nothing.”, Robert Bunsen, as quoted in [1]
Youth and Education
Robert Bunsen was born as the youngest of four sons of the literature professor and librarian Christian Bunsen and Melanie Heldberg, from a family of lawyers. The literature contains different information about Robert Bunsen’s date of birth. While Bunsen’s baptismal entry and a handwritten curriculum vitae refer to March 30, 1811, several reference works mention March 31 as the date of birth, on which Bunsen, according to his biographer Georg Lockemann, also celebrated his birthday in later years. After finishing school in Göttingen and finishing high school in Holzminden, he studied natural sciences, with a focus on chemistry and mathematics at the University of Göttingen. He received his PhD in 1831 with a dissertation Enumeratio ac descriptio hygrometrorum quae inde a Saussurii temporibus proposita sunt on hygrometers. From 1832 to 1833 he travelled through Western Europe on a scholarship from the state government to further broaden his education. During this time he met Friedlieb Ferdinand Runge and Justus Liebig in Gießen as well as Eilhard Mitscherlich in Berlin.[7]
Habilitation and Dangerous Research
Bunsen habilitated in Göttingen in 1834 and began experiments on the (in)solubility of metal salts of arsenic acid. His discovery of iron oxide hydrate is still used today as an antidote to arsenic poisoning. After the death of Friedrich Stromeyer (1835) and before the appointment of Friedrich Wöhler (1836) to the chair of chemistry at the University of Göttigen, Bunsen temporarily took over the chair. In 1836 Bunsen succeeded Friedrich Wöhler at the Höhere Gewerbeschule (Polytechnic) in Kassel. There he began research into cacodyl compounds (tetramethyldiarsan As2(CH3)4 and derivatives), whereby he was injured and partially blinded by a violent explosion in the right eye as early as 1836. In 1838 Bunsen undertook fundamental physical and chemical investigations of the processes taking place in the blast furnace (e.g. blast furnace gas) in the then important ironworks north of Kassel in Veckerhagen. During his first work on blast furnaces, Bunsen discovered that 75% of the calorific value of coal was not used. In England, Bunsen carried out investigations on English blast furnaces with Playfair in 1847. He found that only 20% of the carbon monoxide was used for the reduction process and the majority escaped unused from the blast furnace. He made suggestions on how the heat could be used better.
Gas Analytical Methods and the Zinc-Carbon Battery
In 1839 Robert Wilhelm Bunsen was transferred to the University of Marburg, where he continued his work on cacodyl compounds and the development of gas analytical methods, a work that brought him quick and wide recognition. In 1841 Bunsen developed a zinc-carbon battery (Bunsen element), which was inexpensive and versatile. When the Icelandic volcano Hekla erupted again in 1845, the Danish government invited him on an expedition to Iceland. After his cousin Robert Louis Karl Bunsen, the Elector’s personal physician in Kassel, was able to convince Prince Elector Friedrich Wilhelm in 1846, he was granted six months’ leave. On Iceland Bunsen examined the Great Geyser, where he identified hydrogen, hydrogen sulphide and carbon dioxide in the escaping gases. For the occurrence of hydrogen Bunsen found the explanation of the splitting of hydrogen sulfide into sulfur and hydrogen. Furthermore, he investigated eruptive rocks and feldspars from Iceland to learn their chemical composition.
The most Modern Chemical Laboratory in Germany
In 1850 Bunsen accepted a position at the University of Wroclaw. A new laboratory was built here, and he also met the physicist Gustav Robert Kirchhoff. However, Bunsen taught in Breslau only for three semesters and then followed a call to Heidelberg. In 1852 Bunsen took over the chair from Leopold Gmelin at the Ruprecht-Karls-University. Here Bunsen also received a new laboratory and an official residence. The laboratory was considered the most modern chemical laboratory in Germany. In his experiments Bunsen succeeded in obtaining numerous metals such as chromium, magnesium, aluminium, manganese, sodium, barium, calcium and lithium in elemental form by electrolysis of molten salts. In his collaboration with Sir Henry Roscoe, the light-induced formation of hydrogen chloride from hydrogen and chlorine was investigated starting in 1852.
The Bunsen Burner
After seven years, Bunsen broke off his collaboration with Roscoe in 1859 and worked with Kirchhoff on the spectral analysis of chemical elements. With the help of spectroscopy, the characteristic spectral lines could be examined when chemical substances were heated in flames. For this purpose Bunsen perfected a special gas burner, which had previously been invented by Michael Faraday and was later given the name Bunsens. The Bunsen burner was initially operated with city gas and an addition of oxygen. In the lower part of the flame cone he was able to reduce mineral salt samples (for example bismuth salt to elemental bismuth), in the upper part of the flame the sample was oxidized (bismuth salt to white bismuth oxide).
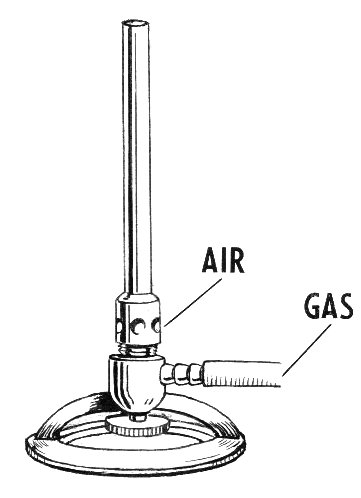
Schematic drawing of a Bunsen Burner
Bunsen has also developed the first low-cost power source, the zinc-carbon element, for laboratory use. Bunsen disassembled the light with a prism and studied the light effect of the disassembled radiation on chemical reactions, plant growth and thought about the light energy between the equator and the Arctic Circle. The spectroscope consisted of a prism with two lenses and an eyepiece in a wooden box. The prism split the uniform white light into a spectrum. If a salt sample was placed in a Bunsen burner flame (a candle flame did not produce good results), the spectroscope showed characteristic colour lines (emission spectra) for each element at certain points of the spectrum. Alkaline and alkaline earth salts as well as indium, thallium and hydrogen could be detected with the spectroscope. Bunsen and Kirchhoff found two new chemical elements in the spectral apparatus: Rubidium and caesium. The detection of elements – even in the smallest traces – in a substance sample was now easily possible.
Later Years
In 1860/61 Bunsen and Kirchhoff discovered the alkali metals caesium and rubidium during the spectral analysis of the mineral water of the newly developed Maxquelle spring in Dürkheim. Their studies also made it possible to explain the Fraunhofer lines and thus lay one of the most important foundations for modern astronomy. Bunsen did not offer any special training in organic chemistry, which was sometimes criticized. However, he employed up to eight other lecturers who offered individual courses on organic and pharmaceutical chemistry, chemical technology, crystallography, forensic chemistry and the history of chemistry. Soldering tube exercises completed the offer. This broad range made Heidelberg attractive for students from other German and European countries and even from overseas.
When Bunsen retired at the age of 78, he devoted himself to geology, which until then had been his hobby. Robert Wilhelm Bunsen died in Heidelberg on August 16, 1899 at the age of 88.
The Bunsen Burner: Where did it come from? | Stuff of Genius, [9]
References and Further Reading:
- [1] Prof. Dr. Ostwald: Gedenkrede auf Robert Bunsen. Aus: Gesammelte Abhandlungen. hg. im Auftrage der Bunsen-Gesellschaft für angewandte physikalische Chemie. 1. Band. Leipzig: Wilhelm Engelmann. 1904. S. LIX.
- [2] Gasometry: Comprising the Leading Physical and Chemical Properties of Gases by Robert Bunsen; translated by Henry Roscoe. London: Walton and Maberly, 1857
- [3] Robert Wilhelm Bunsen at NNDB
- [4] Robert Wilhelm Bunsen at Mathematics Genealogy Project
- [5] Robert Bunsen, German chemist, at Britannica Online
- [6] Gustav Kirchhoff and the Fundamentals of Electrical Circuits, SciHi Blog, March 12, 2014.
- [7] Justus von Liebig and the Agricultural Revolution, SciHi Blog, May 12, 2013.
- [8] Robert Bunsen at Wikidata
- [9] The Bunsen Burner: Where did it come from? | Stuff of Genius, HowStuffWorks @ youtube
- [10] Timeline for Robert Bunsen, via Wikidata





