
J. J. Thomson (1856 – 1940)
On April 30, 1897, English physicist Joseph John Thomson gave the first experimental proof of the electron, which had been already theoretically predicted by Johnstone Stoney. Thomson was awarded the 1906 Nobel Prize in Physics for the discovery of the electron and for his work on the conduction of electricity in gases.
“As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively electrified, and are acted on by a magnetic force in just the way in which this force would act on a negatively electrified body moving along the path of these rays, I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter.”
– J. J. Thomson, “Cathode rays” Philosophical Magazine, 44, 293 (1897).
Background J. J. Thomson
Joseph John Thomson was born in 1856 in Manchester, England and was taught mainly in private schools at the beginning. In 1876, he enrolled at Trinity College, Cambridge where he received his Bachelor’s and Master’s degree. When Thomson became Cavendish Professor of Physics, Ernest Rutherford was among his students and later on, he succeeded Thomson in the post. Thomson was known to be an excellent teacher. Seven of his research assistants and his son were able to win the Nobel Prizes in physics. Thomson himself was awarded the famous prize in 1906 “in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases.” Two years later, he was knighted. In 1918, Thomson became Master of the Trinity College in Cambridge.
The Discovery of the Electron
“The electron: may it never be of any use to anybody!”
– A popular toast or slogan at J. J. Thomson’s Cavendish Laboratory in the first years of the 1900s, as quoted [9]
The fact that atoms were built up from a more fundamental unit was already suggested by scientists like William Prout or Norman Lockyer. However, J. J. Thomson was the first known scientist to suggest that the fundamental unit was over 1000 times smaller than an atom. Today, the subatomic particle is known as the electron. To achieve this discovery, Thomson used his explorations on the properties of cathode rays. He published his suggestion on 30 April 1897 following his discovery that Lenard rays could travel much further through air than expected for an atom-sized particle. At first, he estimated the mass of cathode rays through the heat that was generated when the rays hit a thermal junction. Then Thomson compared his result with the magnetic deflection of the rays.
As a result, Thomson could suggest that cathode rays were more than 100 times lighter than the hydrogen atom and also, he concluded that their mass was the same in whichever type of atom they came from. He then concluded that the rays were composed of light and negatively charged particles, a universal building block of atoms. Thomson named the particles “corpuscles”, but scientists preferred the name electron which had been suggested by George Johnstone Stoney prior to Thomson’s discovery. One month after Thomson’s important announcement of the corpuscle he found that he could reliably deflect the rays by an electric field if he evacuated the discharge tube to a very low pressure. By comparing the deflection of a beam of cathode rays by electric and magnetic fields he obtained more robust measurements of the mass to charge ratio that confirmed his previous estimates. This became the classic means of measuring the charge and mass of the electron. Thomson believed that the corpuscles emerged from the atoms of the trace gas inside his cathode ray tubes. He concluded that atoms were divisible, and that the corpuscles were their building blocks. To explain the overall neutral charge of the atom, he proposed that the corpuscles were distributed in a uniform sea of positive charge. His model became widely known as the “plum pudding” model of atoms. Ernest Rutherford disproved this model later on with his famous gold foil experiment, which led to the discovery of the nucleus.[4]

J. J. Thomson’s cathode ray tube with electromagnetic deflection coils
Discovering Evidence for Isotopes
Next to this famous discovery, Thomson and his research assistant F. W. Aston channeled a stream of neon ions through a magnetic and an electric field. They measured its deflection and observed two patches of light on the photographic plate, which was placed in the path. They concluded, that neon is composed of atoms of two different atomic masses (isotopes), which was the first evidence for isotopes of a stable element. Also, Thomson’s separation of neon isotopes by their mass was the first example of mass spectrometry. In 1905, Thomson discovered the natural radioactivity of potassium and one year later he managed to demonstrate that hydrogen had only a single electron per atom.
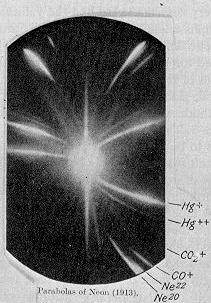
In the bottom right corner of this photographic plate are markings for the two isotopes of neon: neon-20 and neon-22.
Impact
In 1884 Thomson was elected a Fellow of the Royal Society, which awarded him the Royal Medal in 1894, the Hughes Medal in 1902 and the Copley Medal in 1914. In 1902 he was elected to the American Academy of Arts and Sciences, in 1903 to the National Academy of Sciences. In 1906 he was awarded the Nobel Prize in Physics for his research on the electrical conductivity of gases. Thomson was awarded a Knight Bachelor’s degree in 1908 and was admitted to the Order of Merit in 1912. Since 1907 he was a corresponding member of the Bavarian Academy of Sciences. In 1911 he was elected as an external member of the Göttingen Academy of Sciences.
From 1918 until his death in 1940 he was Head of Trinity College and from 1916 to 1920 President of the Royal Society . Thomson’s ashes were buried at Westminster Abbey (near Sir Isaac Newton).
Kathy Joseph, J. J. Thomson Cathode Ray Tube Experiment: the Discovery of the Electron, [10]
References and Further Reading:
- [1] The Discovery of the Electron
- [2] Thomson’s discovery of the isotopes of Neon
- [3] J. J. Thomson’s Nobel Lecture
- [4] Ernest Rutherford Discovers the Nucleus, SciHi Blog
- [5] J. J. Thomson at Wikidata
- [6] Works by or about J. J. Thomson at Internet Archive
- [7] A short film of Thomson lecturing on electrical engineering and the discovery of the electron (1934)
- [8] J.J. Thomson (1897), Cathode rays, Philosophical Magazine, 44, 293
- [9] Proceedings of the Royal Institution of Great Britain, Volume 35 (1951), p. 251.
- [10] Kathy Joseph, J. J. Thomson Cathode Ray Tube Experiment: the Discovery of the Electron, Kathy Loves Physics & History @ youtube
- [11] Rayleigh (1941). “Joseph John Thomson. 1856-1940”. Obituary Notices of Fellows of the Royal Society. 3 (10): 586–609.
- [12] “Joseph John “J. J.” Thomson”. Science History Institute. June 2016.
- [13] Thomson, J. J. (June 1906). “On the Number of Corpuscles in an Atom”. Philosophical Magazine. 11 (66): 769–781
- [14] Thomson, J. J. (1905). “On the emission of negative corpuscles by the alkali metals”. Philosophical Magazine. Series 6. 10 (59): 584–590.
- [15] Timeline for J. J. Thomson, via Wikidata





